Navicular stress fractures & the phases of bone remodelling
This week we are joined by Josh Farragher, Physiotherapist at Physica Spinal and Physiotherapy Clinic in Melbourne, Australia. Josh completed his undergraduate degree at Monash University in 2012 and has since been practicing at Physica. In 2015, Josh completed the graduate certificate of musculoskeletal physiotherapy at La Trobe University and is currently undertaking the masters program at La Trobe.
Over the past few years Josh has developed a great interest in spinal pathologies, particularly non-specific neck pain and is planning to one day further research for physiotherapists in this area. Teaching is another great passion of his and in the future Josh's hope is to help educate the next generation of clinicians. Recently Josh has been learning about navicular stress fractures, and for this blog, he shares his new knowledge about the causes, risk factors, pathophysiology and management of this condition. It's awesome to have other like-minded clinicians put time into sharing their knowledge with others and contributing to Rayner & Smale.
Prevalence of navicular stress fractures
Image courtesy of Google Images
Navicular stress fractures are an overuse injury characterized by tenderness over the navicular bone; which usually results from repetitive impact loading causing a reduction in its load-absorbing properties. As a result, patients presenting with this injury are classically running athletes (Gross & Nunley, 2014), with one study demonstrating 59% of navicular stress fractures are track and field athletes (Brukner et al., 1996).
Navicular stress fractures were first described by Towne and colleagues in 1970 and initially were thought to be rare (Jones & Amendola, 2006). However, we now know navicular stress fractures are one of the most common stress fracture sites in the body (Mann & Pedowitz, 2009), with evidence suggesting they comprise 14% to 25% of all stress fractures (Bennell, Malcolm, Thomas, Wark, & Brukner, 1996; Brukner, Bradshaw, Khan, White, & Crossley, 1996).
Physiology of Bone Stress Response
The navicular bone is anatomically located in a unique position that exposes it to various forces and an unusual vascular supply, leaving it vulnerable to overuse injury (Jones & Amendola, 2006; Mann & Pedowitz, 2009). During foot-strike initially the navicular accepts force from first and second metatarsocuneiform joints, then medially sharing force with the talar head and finally lateral forces from the second metatarsal and middle cuneiform (Gross & Nunley, 2014). As a consequence, the maximum shear stresses are exerted through the central third of the navicular body (Gross & Nunley, 2014; Mann & Pedowitz, 2009).
Through repetitive bouts of mechanical load, potential for bone strain occurs (Warden, Burr, & Brukner, 2006). Repetitive stress of a bone is naturally associated with ‘microdamage’ and is a normal phenomenon in humans (Bennell, Malcolm, Wark, & Brukner, 1996; Warden et al., 2006). Whilst, this is defined as the initial stage of the stress fracture continuum, no current evidence suggests intervention is required as bone has the ability to remodel and repair (Warden et al., 2006). However, repetitive stress associated with failed bone remodelling can begin the pathological process resulting in stress reaction, stress fracture and subsequent complete fracture of the navicular (McCormick et al., 2011; Warden et al., 2006).
The pathological continuum of navicular stress fractures begins when the ‘microdamage’ exceeds the bone’s threshold (Bennell, Malcolm, Wark, et al., 1996). The damage accumulates through cyclic loading when bone remodelling is given insufficient time to repair damage from loading (Warden et al., 2006). However, Romani, Gieck, Perrin, Saliba, and Kahler (2002) suggest the cyclic load to produce failure is significant and not easily attained. Mechanical load may not be the only cause of stress fractures. A study by Kelly and Bronk (1990) found that venous restriction of blood flow initiated bone remodelling.
Whilst the precise pathophysiology of stress fractures is unknown (Warden et al., 2006), Otter, Qin, Rubin, and McLeod (1999) proposed that bone ischaemia results from perfusion and reperfusion of bone after repetitive load. Further to this, Romani et al. (2002) proposed during repetitive loads over long periods, such as long distance running, vascular supply may be diminished with increased pressure and cut off temporarily causing ischaemia in the cells, thus eliciting the remodelling process. Repeated force to the capillaries supplying the navicular are also susceptible to ‘microdamage’; as neutrophils work to repair the damage blood supply can be further restricted, therefore further increasing remodelling (Otter et al., 1999). The ischaemia prompts remodelling where osteocytes network within the bone eventually breaking down the cortex, resulting in weakening of the bone and reducing its ability to cope with subsequent loads (Romani et al., 2002).
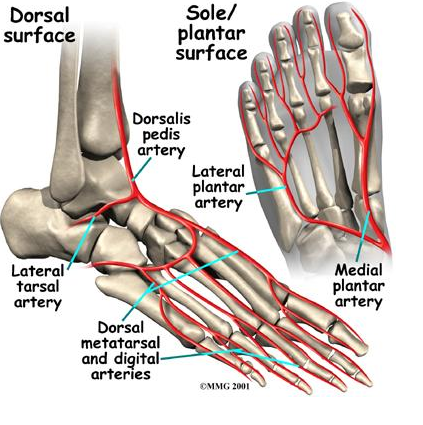
Image courtesy of Google Images
The vascular supply to the navicular consists of branches of the dorsalis pedis artery and the medial plantar artery supplying the lateral and medial third respectively (Mann & Pedowitz, 2009). The central third of the navicular is relatively hypovascular compared with the non-articular surfaces of the bone (Oddy & Davies, 2009). Thus, the risk of oxygen deficiencies in combination with maximal shear forces exerted on this location leave it susceptible to injury (Gross & Nunley, 2014; Oddy & Davies, 2009; Romani et al., 2002). This corresponds with the most common fracture site of the navicular (Mann & Pedowitz, 2009).
Continued loading above the bones threshold would result in progression of the stress continuum eventually resulting in a stress reaction (Bennell, Malcolm, Wark, et al., 1996; Warden et al., 2006). A stress reaction involves clinical symptoms, usually in the early stages, accumulation of microdamage beyond that of bone strain, no visible fracture line on radiology but increased signal uptake on MRI and bone scan (Burne et al., 2005). If the same force is continued further breakdown of the cortex of the bone will occur and eventually formation of a fracture line will appear on radiology signifying the progression to a stress fracture (Burne et al., 2005; Romani et al., 2002; Warden et al., 2006).
Risk Factors
Many risk factors, both intrinsic and extrinsic, are understood to contribute to the likelihood of developing navicular stress fractures (Table 1) (Pegrum, Crisp, & Padhiar, 2012).
Management
Management of stress reactions and stress fractures should be organised around 3 phases to take advantage of the physiological healing process of bone (Romani et al., 2002). Phase I should involve a period of rest to allow maturing of the periosteum, phase II should include general conditioning and strengthening of the injured extremity and phase III should incorporate graded return to activity to allow for further remodelling of the bone (Romani et al., 2002).
Phase I
The primary objective during phase I is to remove stress from the injured area. During this phase osteoclasts secrete proteolytic enzymes creating longitudinal tunnels known as haversian canals, which are aligned with the stress placed on the bone (Romani et al., 2002). The haversian canals are filled with osteoblasts that create a mineralised matrix within the channel and the rest is filled with lamellar bone (Romani et al., 2002). After a week the lamellar bone matures into osteocyte cells, however the periosteum does not mature until around 20 days after remodelling begins (Romani et al., 2002; Wohl, Towler, & Silva, 2009).
During phase I conservative non-weight bearing (NWB) management is the standard of care for initial treatment of partial and complete non-displaced navicular stress fractures (Fowler, Gaughan, Boden, Pavlov, & Torg, 2011; Torg, Moyer, Gaughan, & Boden, 2010). Khan et al (1992) demonstrated an 86% success rate for patients who were treated initially with 6 weeks of NWB cast immobilisation. The same success rate was found when NWB cast immobilisation was used as a second line treatment after 6 weeks of limited activity failed (Khan et al., 1992). Whilst with less participants, Torg et al (1982) found a 100% success rate of immobilisation for 6-8 weeks in a NWB cast. Therefore, the literature suggests the most successful form of management is NWB cast immobilisation for 6 weeks. This 6-week period is recommended regardless of the duration of injury (Fowler et al., 2011; Gross & Nunley, 2014; Oddy & Davies, 2009).
Some researchers recommend follow-up radiology to help determine if the patient should begin weight bearing tasks (Gross & Nunley, 2014), however Potter et al. (2006) found that at a 10 year follow-up some CT scans demonstrated a persistent cleft at the fracture site. Other researchers suggest clinical tenderness of the ‘N’ spot to be the best guide to gauge fracture healing and persistent tenderness should result in further immobilisation (Khan et al., 1994; Oddy & Davies, 2009; Potter et al., 2006). The best evidence suggests pain-free walking and no tenderness over the ‘N’ spot to be the best indicators to progress weight bearing tasks (Fowler et al., 2011; Torg et al., 2010).
Phase II
During phase II osteocyte maturation begins (Romani et al., 2002). Within this phase patients are instructed to progress their weight bearing activities within a pain-free intensity; this is to allow stress to facilitate normal bone remodelling (Romani et al., 2002). With initial loading there is histological evidence of further osteogenesis as the periosteum becomes thickened due to blood vessels within the periosteum enlarging to increase cell proliferation (Romani et al., 2002; Wohl et al., 2009). New osteoblasts begin to form within the already woven bone within a few days (Wohl et al., 2009). After approximately 7 days the thickened periosteum has reduced, the woven bone remains porous with osteoblasts for further remodelling and osteocytes have begun to form and will mature over the coming weeks (Romani et al., 2002; Wohl et al., 2009). Strengthening exercises are progressed throughout this phase increasing in intensity towards functional exercises to aid return to pre-injury activity (Gross & Nunley, 2014; Mann & Pedowitz, 2009). The progression is made on the basis of the patients symptoms (Romani et al., 2002), however researchers suggest running should not be commenced prior to 8 weeks post immobilisation (Gross & Nunley, 2014; Mann & Pedowitz, 2009).
Phase III
Phase III involves increasing functional exercise with the ideal to return to pre-injury function. The exercises involved are therefore based around running, jumping and change of direction depending on the patient’s intended function (Gross & Nunley, 2014; Jones & Amendola, 2006; Romani et al., 2002). During this phase, formation of trabecular channels occurs with functional loading and remodelling, involving primarily osteocyte and periosteal maturation with adequate rest (Romani et al., 2002). Currently the most comprehensive study demonstrated a return to sporting activities in an average of 5.6 months after 6 weeks of NWB immobilisation (Khan et al., 1992). However, with MRI becoming more accessible early detection may help reduce return to sport times and ultimately reduce morbidity associated with navicular stress fractures.
Josh
Conclusion
The key message that I took from reading this piece is that stress fractures are common in the running population and take several months to heal well. Many clients will need coaching and guidance to get through their recovery and the phases presented above provide clear goals. They are always helpful for clinicians to educate their clients about the importance of NWB, having realistic expectations about healing timeframes and also address predisposing risk factors that may contribute to further injury.
An excellently written blog by Josh, thank you again for your contribution - Sian
References
Bennell, K. L. M., Crossley, K. A. Y., Jayarajan, J., Walton, E., Warden, S., Kiss, Z. S., & Wrigley, T. I. M. (2004). Ground Reaction Forces and Bone Parameters in Females with Tibial Stress Fracture. Medicine & Science in Sports & Exercise, 36(3), 397-404.
Bennell, K. L. M., Malcolm, S. A., Thomas, S. A., Wark, J. D., & Brukner, P. D. (1996). The Incidence and Distribution of Stress Fractures in Competitive Track and Field Athletes. The American Journal of Sports Medicine, 24(2), 211-217.
Bennell, K. L. M., Malcolm, S. A., Wark, J. D., & Brukner, P. D. (1996). Models for the pathogenesis of stress fractures in athletes. British Journal of Sports Medicine, 30(3), 200-204.
Brukner, P. D., Bradshaw, C., Khan, K. M., White, S., & Crossley, K. (1996). Stress Fractures: a review of 180 cases. Clinical Journal of Sports Medicine, 6(2), 85-89.
Burne, S. G., Mahoney, C. M., Forster, B. B., Koehle, M. S., Taunton, J. E., & Khan, K. M. (2005). Tarsal navicular stress injury: long-term outcome and clinicoradiological correlation using both computed tomography and magnetic resonance imaging. American Journal of Sports Medicine, 33(12), 1875-1881.
Field, A. E., Gordon, C. M., Pierce, L. M., Ramappa, A., & Kocher, M. S. (2011). Prospective study of physical activity and risk of developing a stress fracture among preadolescent and adolescent girls. Archives of Pediatrics & Adolescent Medicine, 165(8), 723-728.
Fowler, J. R., Gaughan, J. P., Boden, B. P., Pavlov, H., & Torg, J. S. (2011). The Non-Surgical and Surgical Treatment of Tarsal Navicular Stress Fractures. Sports Medicine, 41(8), 613-619.
Gross, C. E., & Nunley, J. A. (2014). Medial-sided Stress Fractures: Medial Malleolus and Navicular Stress Fractures. Operative Techniques in Sports Medicine, 22(4), 296-304.
Harrast, M. A., & Colonno, D. (2010). Stress fractures in runners. Clinics in Sports Medicine, 29(3), 399-416. Jones, M. H., & Amendola, A. S. (2006). Navicular stress fractures. Clinics in Sports Medicine, 25(1), 151-158.
Kelly, P. J., & Bronk, J. T. (1990). Venous Pressure and Bone Formation. Microvascular Research, 39(3), 364-375.
Khan, K. M., Brukner, P. D., Kearney, C., Fuller, P. J., Bradshaw, C. G., & Kiss, Z. S. (1994). Tarsal navicular stress fracture in athletes. Sports Medicine, 17(1), 65-76.
Khan, K. M., Fuller, P. J., Brukner, P. D., Kearney, C., & Burry, H. C. (1992). Outcome of conservative and surgical management of navicular stress fracture in athletes: eighty-six cases proven with computerized tomography. The American Journal of Sports Medicine, 20(6), 657-666.
Lappe, J. M., Stegman, M. R., & Recker, R. R. (2001). The Impact of Lifestyle Factors on Stress Fractures in Female Army Recruits. Osteoporosis International, 12(1), 35-42.
Mann, J. A., & Pedowitz, D. I. (2009). Evaluation and treatment of navicular stress fractures, including nonunions, revision surgery, and persistent pain after treatment. Foot and Ankle Clinics, 14(2), 187-204.
McCormick, J. J., Bray, C. C., Davis, W. H., Cohen, B. E., Jones, C. P., 3rd, & Anderson, R. B. (2011). Clinical and computed tomography evaluation of surgical outcomes in tarsal navicular stress fractures. American Journal of Sports Medicine, 39(8), 1741-1748.
Milgrom, C., Finestone, A., Segev, S., Olin, C., Arndt, T., & Ekenman, I. (2003). Are overground or treadmill runners more likely to sustain tibial stress fracture? British Journal of Sports Medicine, 37(2), 160-163.
Oddy, M. J., & Davies, M. B. (2009). Stress Fractures of the Navicular. Operative Techniques in Sports Medicine, 17(2), 115-118.
Otter, M. W., Qin, Y. X., Rubin, C. T., & McLeod, K. J. (1999). Does bone perfusion/reperfusion initiate bone remodeling and the stress fracture syndrome? Medical Hypotheses, 53(5), 363-368.
Pegrum, J., Crisp, T., & Padhiar, N. (2012). Diagnosis and management of bone stress injuries of the lower limb in athletes. BMJ, 344, e2511.
Potter, N. J., Brukner, P. D., Makdissi, M., Crossley, K., Kiss, Z. S., & Bradshaw, C. (2006). Navicular stress fractures: outcomes of surgical and conservative management. British Journal of Sports Medicine, 40(8), 692-695.
Romani, W. A., Gieck, J. H., Perrin, D. H., Saliba, E. N., & Kahler, D. M. (2002). Mechanisms and Management of Stress Fractures in Physically Active Persons. Journal of Athletic Training, 37(3), 306-314.
Ross, R. A., & Allsopp, A. (2002). Stress Fractures in Royal Marines Recruits. Military Medicine, 167(7), 560-565.
Shaffer, R. A., Rauh, M. J., Brodine, S. K., Trone, D. W., & Macera, C. A. (2006). Predictors of stress fracture susceptibility in young female recruits. American Journal of Sports Medicine, 34(1), 108-115.
Taunton, J. E., Ryan, M. B., Clement, D. B., McKenzie, D. C., Lloyd-Smith, D. R., & Zumbo, B. D. (2002). A retrospective case-control analysis of 2002 running injuries. British Journal of Sports Medicine, 36(2), 95-101.
Tenforde, A. S., Sayres, L. C., Sainani, K. L., & Fredericson, M. (2010). Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: a review of the literature. PM & R: the journal of injury, function and rehabilitation, 2(10), 945-949.
Torg, J. S., Moyer, J., Gaughan, J. P., & Boden, B. P. (2010). Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. American Journal of Sports Medicine, 38(5), 1048-1053.
Torg, J. S., Pavlov, H., Cooley, L. H., Bryant, M. H., Arnoczky, S. P., Bergfeld, J., & Hunter, L. Y. (1982). Stress fractures of the tarsal navicular. A retrospective review of twenty-one cases. The Journal of Bone and Joint Surgery, 64(5), 700-712.
Warden, S. J., Burr, D. B., & Brukner, P. D. (2006). Stress Fractures: Pathophysiology, Epidemiology, and Risk Factors. Current Osteoporosis Reports, 4(3), 103-109. Wohl, G. R., Towler, D. A., & Silva, M. J. (2009). Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone, 44(2), 320-330.