The Neuromatrix of pain overlaps with the functional neuroanatomy of sleep
This is the fourth and final blog on sleep, all of which have been inspired by taking the Sleep Science course offered by the University of Michigan through Coursera. During the course I learnt one statement "sleep deprivation increases pain sensitivity" and this series of blogs has been a way for me to delve deeper into this statement and understand what research has lead us to know this is true.
Be prepared, this blog definitely gets more complicated and technical, but I felt the need to finish searching for the answers to my questions about sleep and pain. If all you take away from this is that the neuroanatomy of sleep and pain overlaps and sleep and pain are undeniably linked then I will have shared my primary message with you. But if you, like me, want to know the finer detail.... continue with me on this final blog about sleep.
The previous blog explored the research showing how the relationship between pain and sleep is bidirectional. What explains this two-way relationship? Many of the articles suggested it is due to the multiple functions of neurotransmitters in the brain. With a desire to know more about what these authors meant I have continued to look on a deeper level at the overlap in functional areas of the brain involved in sleep and pain. I decided to compare the regions of the brain involved in wake and sleep and the Neuromatrix. Let’s take a closer look at the functional neuroanatomy in the hope of making this complex system slightly more clear.
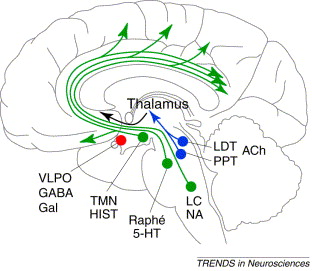
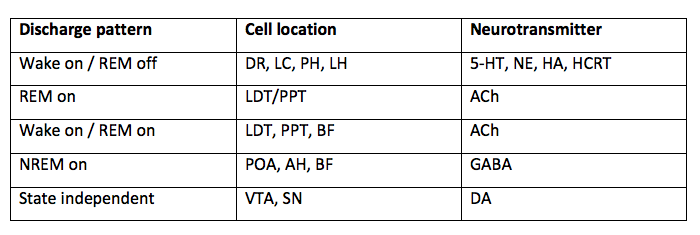
In the first sleep blog I presented the following information. Now I'd like to expand on this in further details. One of the ways to understand neurotransmitter functions is to look at their state-specific discharge pattern and which area of the brain it is released from during each state of sleep and wakefulness.
Main neurotransmitters:
Image courtesy of google images
- Serotonin 5-HT
- Norepinephrine NE
- Histamine HA
- Acetylcholine Ach
- GABA
- Orexin/ Hypocretin HCRT
- Adenosine
Cell locations:
- Dorsal raphe nucleus DR
- Locus coeruleus LC
- Posterior hypothalamus PH
- Anterior hypothalamus AH
- Lateral hypothalamus LH
- Pontine reticular formation PRF/Pons
- Basal forebrain BF
- Tuberomammillary nucleus TMN
- Laterodorsal tegmental area LDT
- Substansia nigra SN
- Ventrolateral pre-optic area VLPO
- Pre-optic nucleus PON
- Median pre-optic nucleus MPN
There are three states of sleep: Wake, REM sleep, and NREM sleep. Depending where in the brain these neurotransmitters are located will alter their action. What you can see from the table above is that the main neurotransmitters regulating sleep & wakefulness are:
- REM: ACh
- NREM: GABA and Adenosine
- Wake: ACh, 5-Ht, NE, HA, dopamine, hypocretin, orexin and glutamate.
REM stands for rapid eye movement, a trait specific to REM sleep that helps us to differentiate between other states of sleep. Compared to NREM sleep and wake, REM sleep displays high levels of rapid eye movement.
A key trait of NREM sleep is low muscle tone and high levels of brain activity.
A key trait of wake is the combination of high muscle tone, brain activity and eye movement. Being awake is not a heterogeneous state which means there are varying degrees of wakefulness which are modulated by these neurotransmitters.
These traits of sleep are similar to other mammals, which is why scientists used animal studies to explore the brain before medical advances created functional MRI, MR brain, ECG, EEG, EMG.
Different brains sizes in mammals, courtesy of google images.
Neuroanatomy of sleep
REM sleep & wake
Norepinephrine (NE), histamine (HA) and serotonin (5-HT) are monoamines involved in wake and REM sleep which have a wake-on, REM-off discharge pattern. Activities that promote NE, 5-HT and HA all promote wakefulness. An easy way to remember this is that exercising makes us feel more awake (increasing serotonin levels) and antihistamine medication can make us drowsy.
Aside from the monoamines there is another neurotransmitter involved in REM & Wake called Acetylcholine (ACh). ACh is a state-specific neurotransmitter meaning that it has different functions depending on which state it is discharged in i.e. wake-on, REM-on. It is also region-specific meaning that depending where it is released from and to will change its action. When it is released in the thalamus, ACh promotes wakefulness but when it is released in the pontine reticular formation it triggers REM sleep. The receptors for ACh found in the forebrain are associated with memory formation and loss of ACh-receptors in this region of the brain is associated with the development of Alzheimer’s disease. Another clinically relevant point is that when people have short REM sleep latency i.e. not spending a long time transitioning from NREM to REM sleep, they generally display cholinergic hyperactivity and therefore medications which manage cholinergic secretion help to regulate sleep and wakefulness.
GABA is another major inhibitory neurotransmitter in the brain and most GABAa receptors or 'gabaergic' drugs such as benzodiazepines are used clinically to produce sleep, sedation and general anaesthetic. GABA is a NREM-on neurotransmitter i.e activation of GABAa and GABAb receptors produce sleep. GABA and ACh are carefully balanced to create normal REM and NREM sleep. GABA has an inhibitory effect on ACh so while it promotes sleep, it actually reduces REM sleep making sleep abnormal, hence why it is a neurotransmitter considered in both REM sleep and NREM sleep. This explains part of the reason why sedation or ‘putting someone to sleep’ is not actually putting them into a normal pattern of sleep.
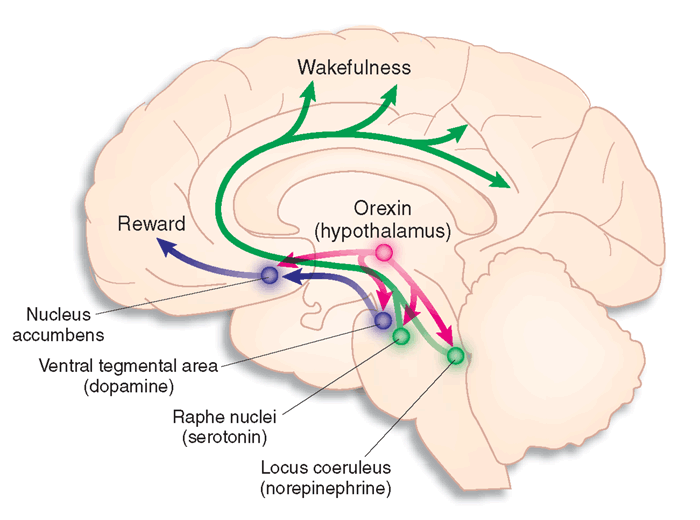
The final neurotransmitter to consider in REM & Wake is Orexin or Hypocretin, which are contained in the lateral hypothalamus. From the LH orexin has projections to the tuberomammillary nucleus, posterior hypothalamus, LC, DR, PRF, medullary reticular formation and laterodorsaltegmental area. Orexin neurones receive input from multiple arousal-regions of the brain and it has projections to regions of the brain where other wake-promoting neurotransmitters live. Orexin promotes wakefulness and helps with the transition from sleep to wakefulness. Orexin levels peak during waking and are involved in promoting motor control and activity. It was only recently discovered and first published in 1998 and is the neurotransmitter known to control appetite. Scientists believe that the sleep disorder narcolepsy is caused by an absence of Orexin in the cerebrospinal fluid.
NREM sleep
There are two main neurotransmitters involved in the generation of NREM sleep which are GABA and Adenosine. It was only after 1986 that scientists reported on the presence of gabaergic neurones in the forebrain, which have a NREM-on discharge pattern. NREM sleep is essential for recovery. GABA and adenosine levels increase prior to NREM sleep and help with the transition from wake to NREM sleep which lead us to believe that these neurotransmitters contribute to the quality of our 'recovery sleep'.
Caffeine is the most widely used psycho-active drug in the world and it promotes wakefulness and cognitive performance by acting as an adenosine receptor antagonist. Adenosine receptors are located in the hypothalamus and pre-optic regions. Adenosine is one of the most important neuromodulators for the homeostasis of sleep drive. The longer we are awake, the higher the levels of adenosine build up in the forebrain which inhibits ACh, NE and other wake promoting neurotransmitters. It is closely related to the circadian rhythm and peaks throughout the day to assist with our drive to fall asleep.
The neuroanatomy of pain
the neuromatrix
There is no single 'pain processing area of the brain', instead there is thought to be a series of neural connections known as the neuromatrix. The pain matrix is thought to consist of the dorsolateral prefrontal cortex, insula cortex, anterior cingulate cortex, primary and secondary sensory cortices, and the thalamus (Moseley, 2008).
Every individual has a unique pain experience (and an unique neuromatrix), but there are some areas of the brain (listed above) which are seen to be more frequently active on brain functional imaging. What surprised me is how many of these areas are involved in the regulation of sleep and are part of the projected pathway of neurotransmitters released from the midbrain and brainstem during sleep and wakefulness.
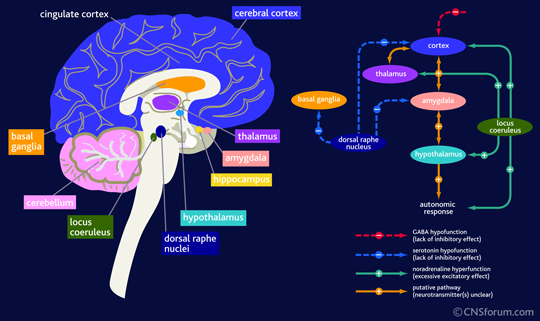
Image courtesy of Google Images, retrieved September 10th 2015 https://www.cnsforum.com/educationalresources/imagebank/brain_struc_anxiety/neuro_biol_gad_2
Image courtesy of Google Images retrieved September 10th 2015 http://www.nature.com/nm/journal/v13/n2/fig_tab/nm0207-126_F1.html
The Locus Coeruleus (LC) is a nucleus in the Pons (brainstem) involved in the physiological responses of stress and pain. It is the primary site for the synthesis of norepinephrine (NE), a major neurotransmitter that promotes wakefulness. It is affected by stress causing an increased secretion of NE to the prefrontal cortex, which results in altered cognitive function, increased motivation, and also an increased secretion of adrenaline.
The prefrontal cortex is part of the frontal lobe and involved in one's personality, specifically relating to planning complex cognitive behaviour, personality expression, decision making, and moderating social behaviour. Opiods inhibit the firing of neurones in the LC and often when opiod medication is ceased, there is a sudden increase in production of NE which is associated with opiod withdrawal.
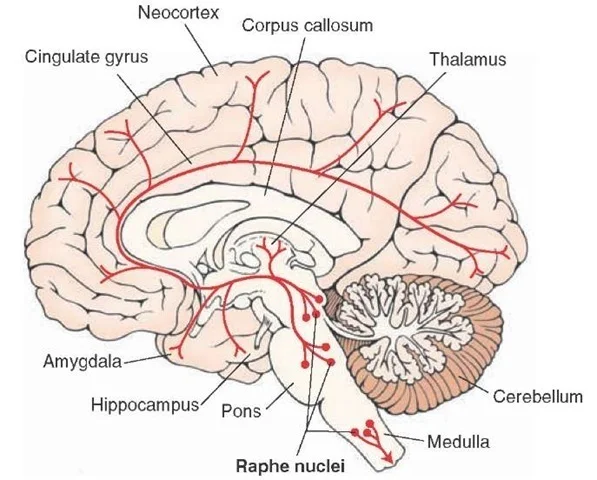
Image courtesy of Google Images retrieved September 10th 2015 http://what-when-how.com/neuroscience/neurotransmitters-the-neuron-part-4/
The Dorsal raphe nucleus (DR) is also found in the brainstem and contains neurones which synthesise serotonin (5-HT), which also has multiple projections to the forebrain. The DR is the site of action of selective serotonin reuptake inhibitors (SSRIs) which are commonly used to treat depression by promoting 5-HT production, which in turn promotes wakefulness. Serotonin also has projections to the amygdala – two almond shaped nuclei located deep within the temporal lobes that perform a primary role in processing of memory, decision making and emotional reactions. The amydala projects to the hypothalamus (HT), dorsomedial thalamus, LC, trigeminocervical nucleus and many other nuclei. The primary role of the amygdala is in the formation and storage of memories associated with emotions, particularly fear.
The Pontine reticular formation (PRF) is a set of nuclei found in the brainstem and is the part of the brain where acetylcholine (ACh) is released. ACh released in this part of the pons is REM sleep promoting. The PFR has many diverse functions in the body - some control posture, balance, muscle tone from projections to the reticulospinal tracts, while others provide cardiovascular control, habituation, sleep and consciousness control and pain control (via the descending analgesic pathways).
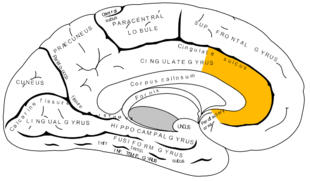
Anterior cingulate cortex (ACC) courtesy of Google Images.
The anterior cingulate cortex is part of the cingulate gyrus and is thought to be responsible for autonomic functions (regulating heart rate and blood pressure) and cognitive functions (reward anticipation, decision making, empathy, impulse control and emotion). Moseley (2003) describes it as the action centre which causes humans to think "what should I do?" In relation to pain, the ACC is thought to provide an emotional description of pain and to coordinate an appropriate response.
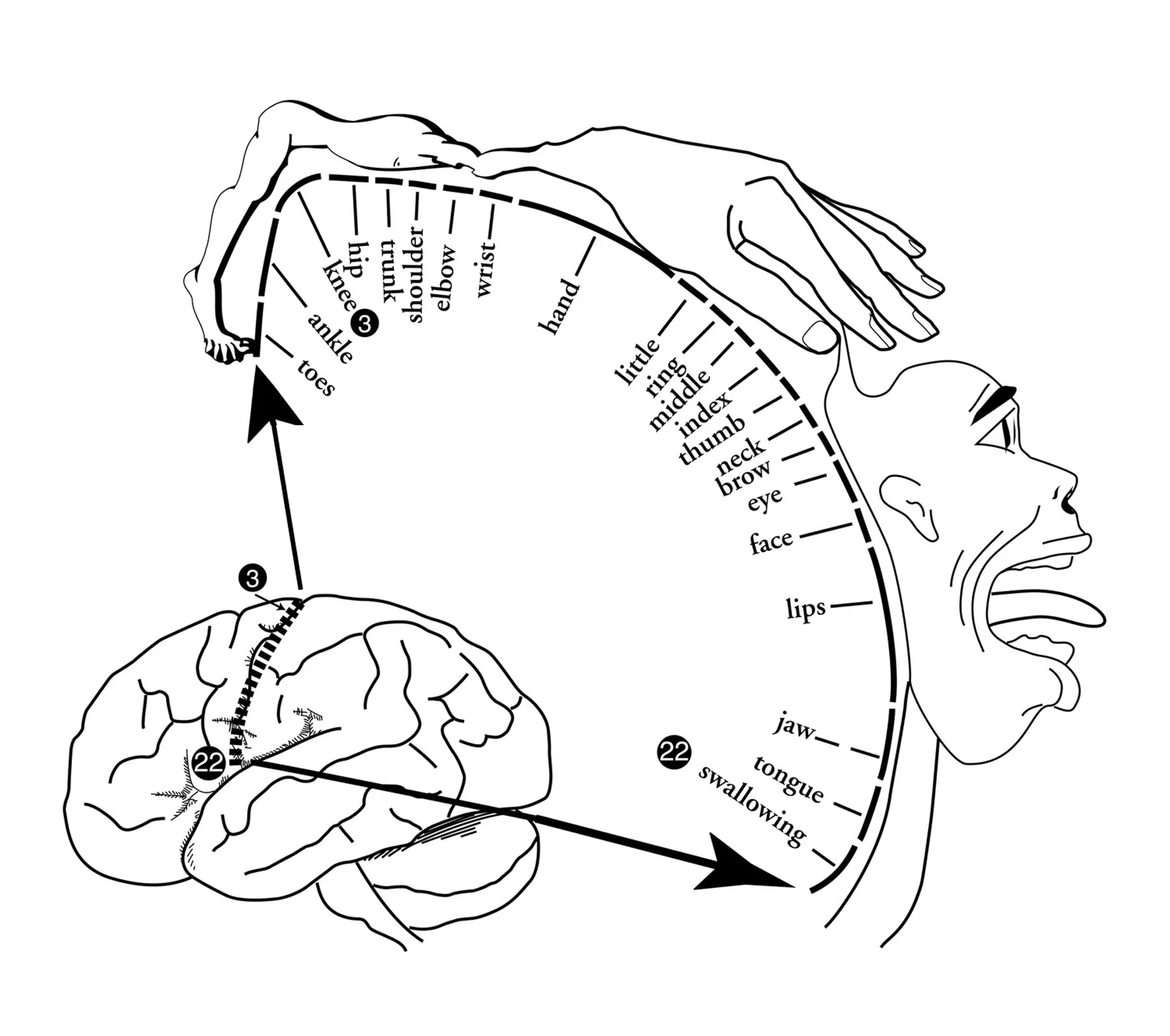
Homonculus - Explain Pain by NOI group, taken from Google Images
The insula cortex is found within the deep folds of the cerebral cortex and is involved in linking emotion to action. These functions may include perception, self-awareness, cognitive function, and motor control i.e it is involved in understanding the physiological functioning of the entire body.
The thalamus functions in relaying sensation, spatial sense and motor signals to the cerebral cortex, along with the regulation of consciousness, sleep and alertness.
The primary and secondary sensory cortices perceive and interpret sensations and touch. When being taught about the somatosensory cortex we learn about the homunculus (the mapping of regions on the body on the brain).
In summary
opefully the image that is emerging from this information is that many parts of the neuroanatomy between pain processing and sleep regulation are linked. Sleep plays a vital role in memory consolidation, mood and personality expression, cognitive function, pain modulation and many other physiological and psychological traits of human functioning. Sleep is vital for our health and clearly has a huge impact on how we feel and function.
The course sparked a curiosity for me about sleep and chronic pain. How much sleep is impacting pain and vice versa might be difficult to objectively determine but clearly they are linked. I don’t know yet the impact that correcting sleep alone can have on pain modulation but, I am confident in saying more attention should be paid to questioning about the quality of sleep and having processes in place that allow for referral to psychologists and sleep doctors to assist with the diagnosis and management of these problems.
What I have taken away from these blogs is that prioritising sleep as a major part of health and balance will infiltrate into every other aspect of our lives.
Sian
References
Butler, D. S., & Moseley, G. L. (2013). Explain Pain:(Revised and Updated. Noigroup Publications.
Moseley, G. L. (2003). A pain neuromatrix approach to patients with chronic pain. Manual therapy, 8(3), 130-140.